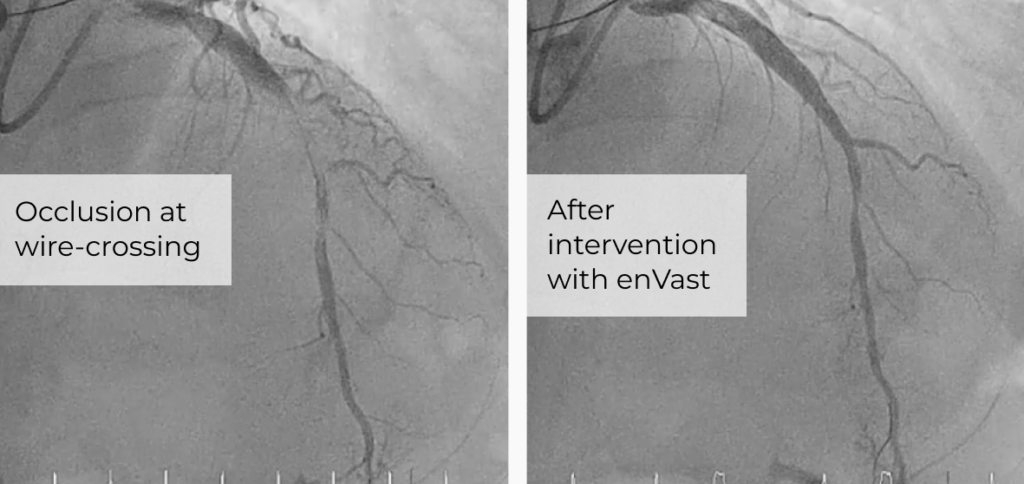
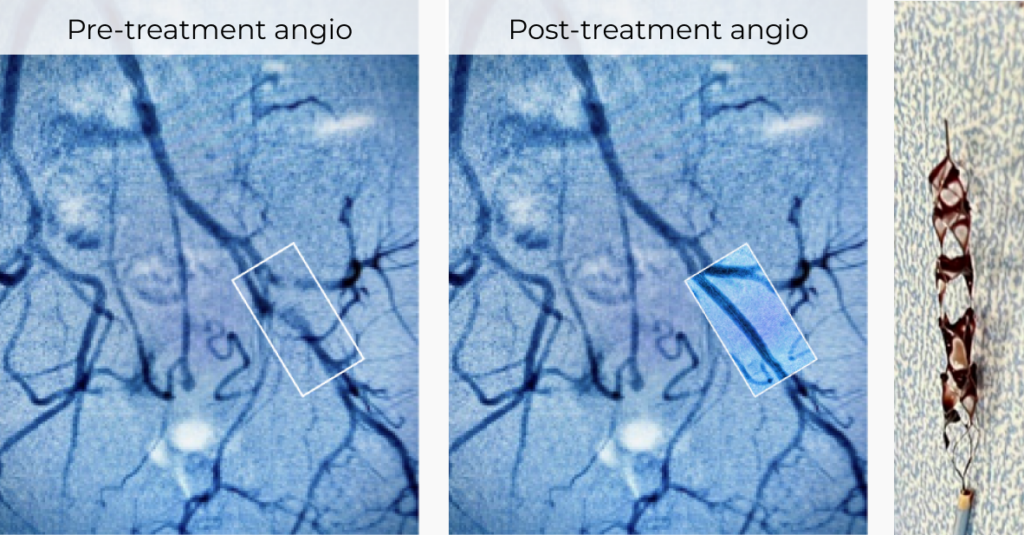
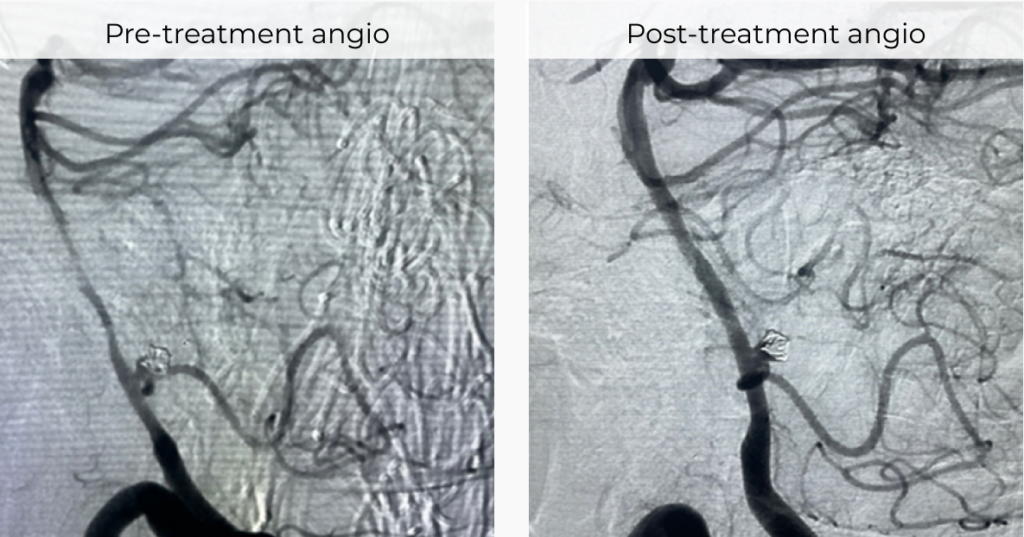
Vesalio Expands International Neurovascular Portfolio with CE Mark of NeVa VS and NeVa 3.0 mm and Receives Additional FDA 510(k) Clearance for its Aspiration Catheters
Company advances global neuro-intervention footprint with new CE-marked devices for vasospasm and stroke, and expanded U.S. indications for aspiration catheters.