The VITAL Trial confirms NeVa VS to be a safe treatment for Cerebral Vasospasm. Click to find out more:
DISCOVER neva vs
A SPECIFIC DESIGN, A DECISIVE SOLUTION for post aSAH Vasospasm
NEVA VS FOR VASOSPASM: RECENT EVIDENCE
Click to watch the presentation highlighting NeVa VS as the game-changer in treating vasospasm after aneurysmal sub-arachnoid haemorrhage (aSAH) at the BRAIN CONFERENCE in London, December 2024.
“NeVa VS is a safe strategy to regain vessel diameter in severely narrowed proximal intracranial arteries”
– Dr. Ameer Hassan, Valley Baptist Neuroscience Institute, Harlingen, Texas
CEREBRAL VASOSPASM
COMMON AND DEADLY
1 in 10000 People experience an aneurysm rupture every year. Up to 70% of these patients have to cope with cerebral vasospasm, the most common complication of aneurysmal Sub arachnoid haemorrhage (a-SAH) as well as the leading cause of delayed morbidity and mortality.
WITH NEW TREATMENT OPTIONS REVERSE VASOSPASM
Cerebral vasospasm is a life-threatening narrowing of brain vessels that usually occurs 7 to 10 days after an aSAH. The severe constriction limits blood flow to the affected area, causing delayed cerebral ischemia and poor neurological outcomes.
Today, with advances in technology and new treatment pathways, cerebral vasospasm could be reversible.
Case images courtesy of Dr Arthur Grigorian, MD, Wellstar North Fulton Medical Center. Roswell, Georgia
NEVA VS, SPECIFIC TO VASOSPASM TREATMENT
NeVa VS combines a smooth and continuous architecture and the deliverability of a stent retriever with an enhanced outward radial force to effectively dilate critically narrowed vessels.
EXPANSION OPTIMIZED
FLOW MAINTAINEDNeVa VS is a patented retrievable nitinol stent specifically designed to treat cerebral vasospasm after aneurysmal rupture. It combines a smooth and continuous architecture and the deliverability of a stent retriever with an enhanced outward radial force to effectively dilate critically narrowed vessels.

SPECIFIC
Designed For Vasospasm
Optimized Vessel Expansion
Distal Flow Maintained During Treatment
SUCCESSFUL
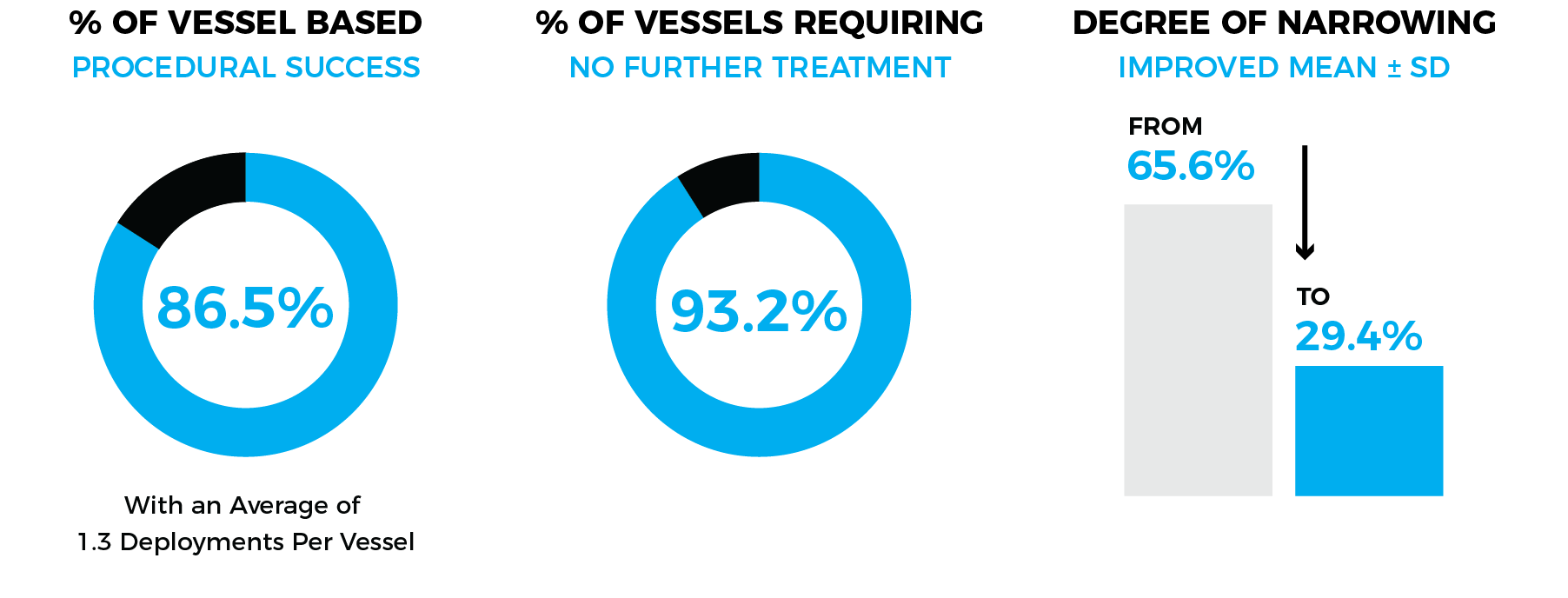
SAFE
No Vessel injuries or Ruptures
NEVA VS
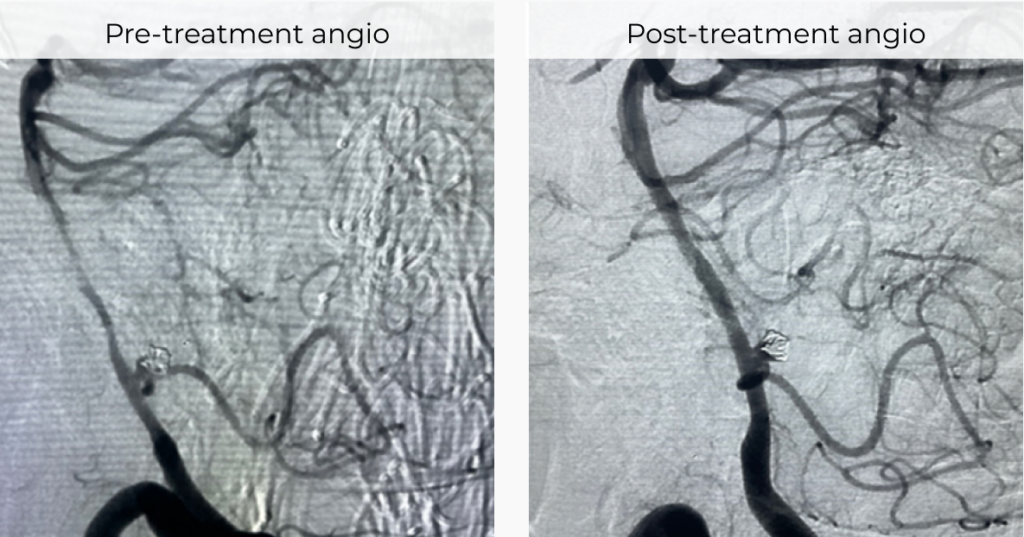
IN ACTIONMODERATE-SEVERE BASILAR ARTERY VASOSPASM SUCCESSFULLY RESOLVED WITH NEVA VS
Dr Ryan M Hebert, Yale School of Medicine, New Haven, Connecticut
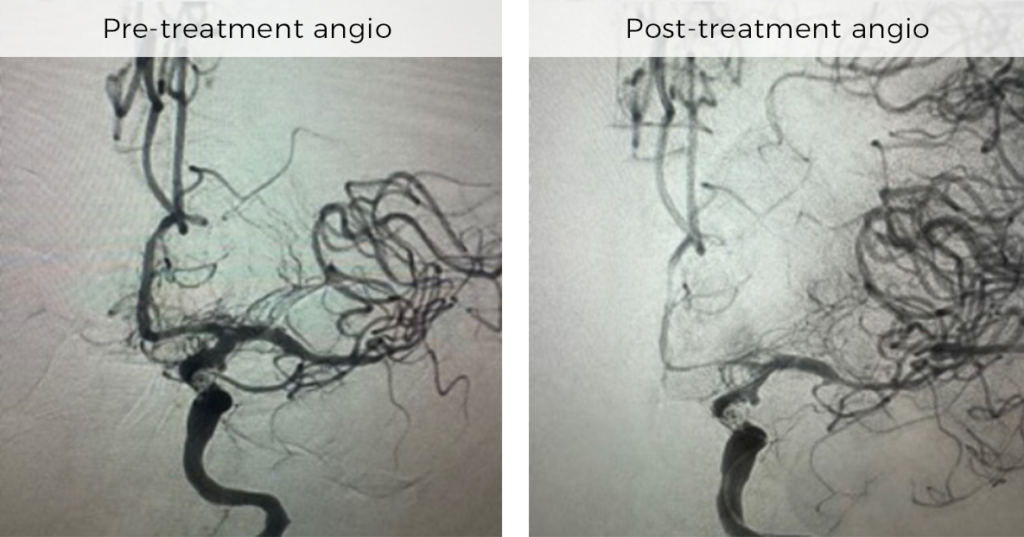
RIGHT MIDDLE CEREBRAL AND ANTERIOR CEREBRAL ARTERY VASOSPASM TREATED WITH NEVA VS
Dr Arthur Grigorian, Wellstar North Fulton Roswell, Georgia
approved for use
in the U.s.
NeVa VS is the first and only FDA HDE approved device for the adjunct treatment of symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage.
Approved by FDA under HDE 210004. Note: The effectiveness of this device for this use has not been demonstrated”