enVastTM
WHEN THE PROBLEM IS CLOT BURDEN
enVast is the first and only CE approved stent retriever for temporary endovascular use to restore blood flow in patients experiencing thrombosis symptoms in the coronary vasculature.
CHOOSE TO REMOVE FOR RAPID REPERFUSION DURING A STEMI HEART ATTACK
The most common type of heart attack, an ST-elevation myocardial infarction (STEMI), is also the most dangerous.
A STEMI is caused by a blood clot, called a large thrombus burden (LTB), that completely blocks an artery to the heart.
When an artery to the heart is blocked, oxygenation is obstructed, leading to heart muscle tissue damage.
NO TIME
TO WASTE
STEMI heart attack patients are two times more likely to die than other heart attack victims. Survivors have a 2 to 4 times higher risk of experiencing major, life-altering adverse events.
Reopening the artery and restoring blood flow as soon as possible is essential for preventing permanent damage or death.
Treatment Window is Short:
- Less than 3 hours from the onset, the patient has a chance of full recovery
- Greater than 3 hours from the onset, the patient will likely have some permanent damage
- Greater than 12 hours, patient outcome is poor
ENDOVASCULAR APPROACHES IN STEMI
Conventional endovascular treatment for STEMI patients involves opening the artery with balloon & stent:
- Outcomes remain poor in ~50% due to residual thrombus in the vessel
Endovascular aspiration continues to be debated. Studies show thrombus aspiration alone:
- does not improve reperfusion or outcomes, and
- bear higher potential for stroke
ENVAST DROP ZONE TECHNOLOGY IN ACTION
ENVAST
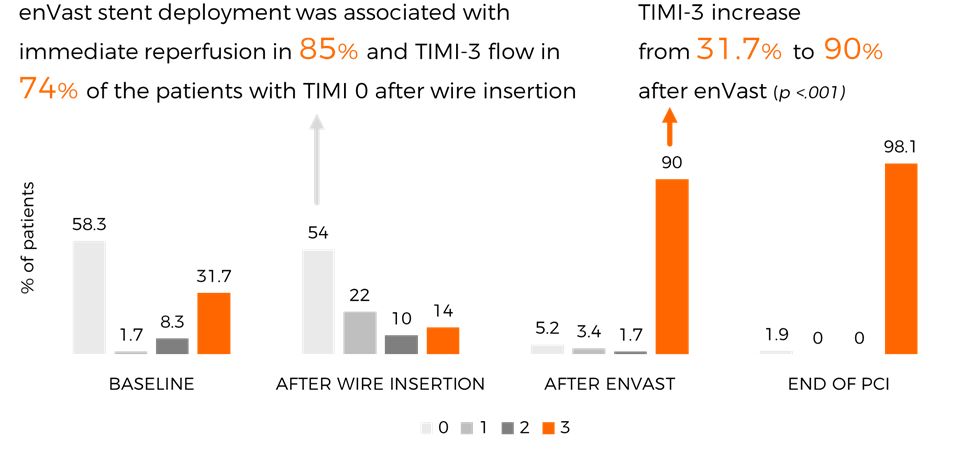
85% IMMEDIATE REPERFUSION
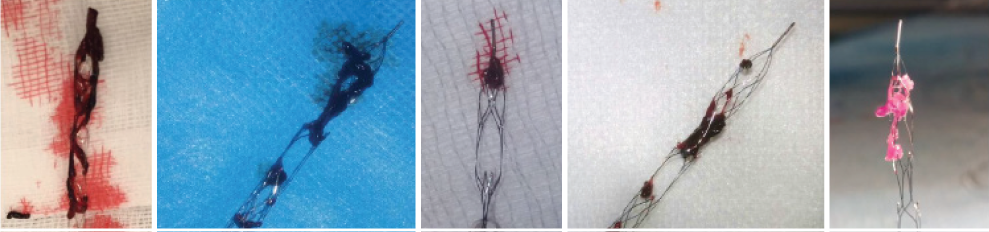
Equipped with Drop Zone technology, the enVast coronary thrombectomy system has been proven to remove large thrombus burden (LTB) and create immediate reperfusion in 85% of cases.
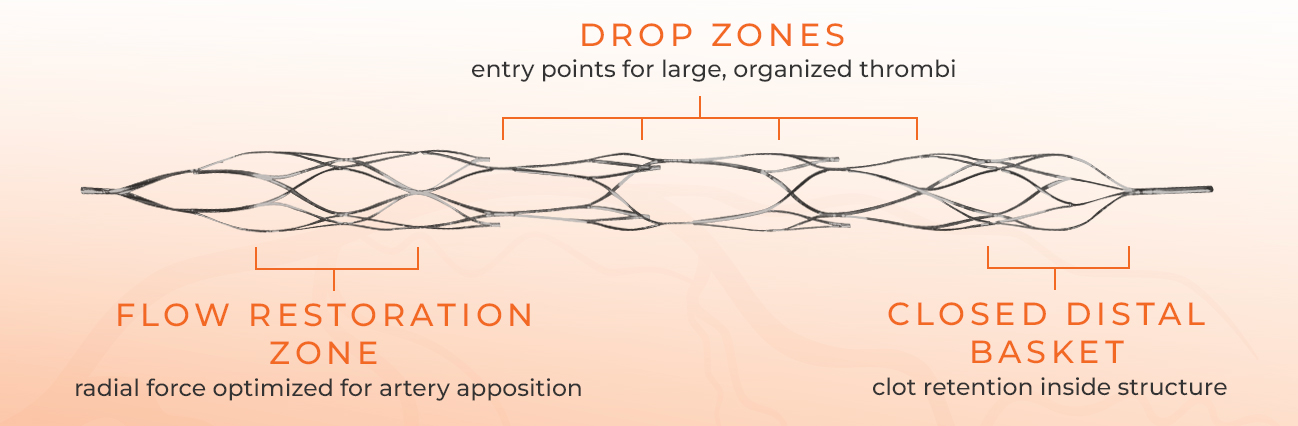
Drop Zone technology captures the thrombus inside the device, and the closed distal tip keeps it from escaping during removal.
Each Drop Zone provides an additional opportunity to secure the clot burden, making first pass success common.
Critical minutes are saved for better patient outcomes.
FIRST–IN–HUMAN
Cases
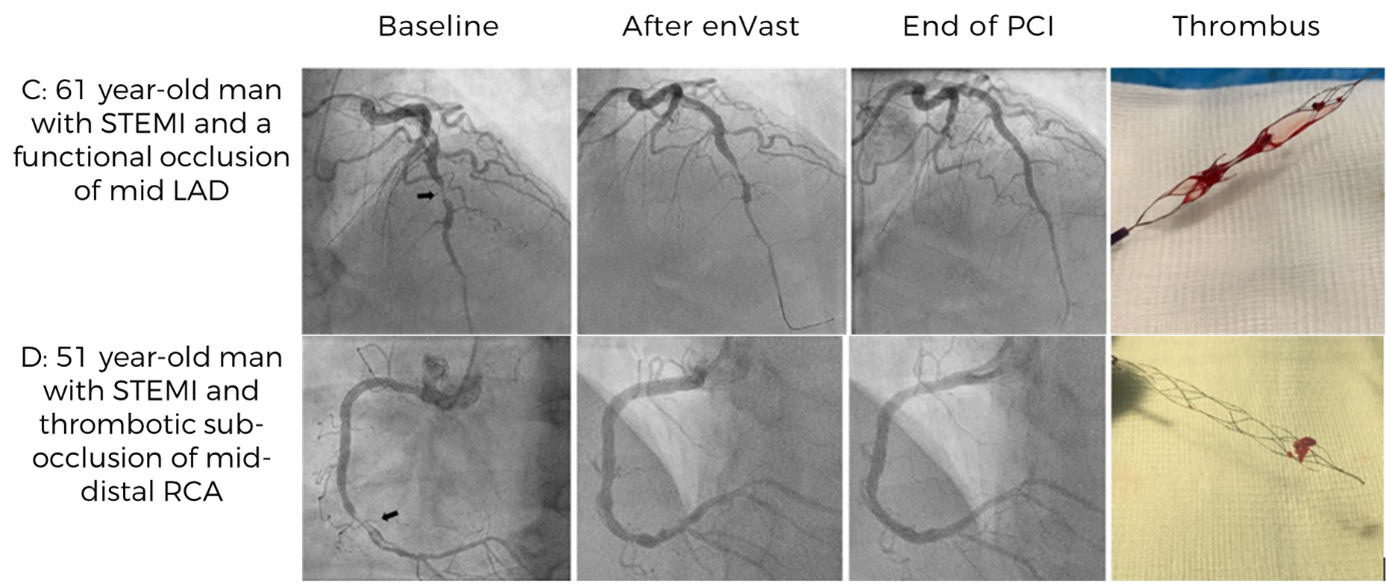
In a first-in-human case series of 61 STEMI patients with LTB, a thrombectomy technique with an enVast stent retriever and continuous aspiration was developed for coronary care.
Safe & Successful
The technique was safe and warranted high rates of successful flow restoration in culprit vesselsNO ADVERSE EVENTS
Procedural complications (i.e., coronary spasms) were mild and reversible, and no serious adverse events were reported.

ENVAST TECHNOLOGY
IN ACTION
Dr. Marco Vaglimigli, Deputy Chief of Cardiology at Cardiocentro Ticino Istituto, Switzerland, uses enVast in STEMI patients
In mid-2022, Vesalio began a clinical trial for STEMI patients.
The NATURE study, a randomized, multi-center clinical trial, will compare the safety and efficacy of enVast™ as an adjunctive measure to conventional intervention versus the standard of care in STEMI patients with an LTB.
ENVAST PORTFOLIO
ADAPTS TO ANATOMIES
enVast is offered in several sizes to adapt to the anatomical challenges presented by STEMI patients
CHOOSE
TO REMOVE
enVast devices with freshly removed LTB clots inside