Paper:
Primary results from the CLEAR study of a novel stent retriever with Drop Zone technologyAuthors:
Albert J Yoo, Serdar Geyik, Michael T Froehler, Christoph Johannes Maurer, Tareq Kass-Hout, Osama O Zaidat, Raul G Nogueira, Ricardo A Hanel,
Laurent Pierot, Laurent Spelle, Demetrius Lopes, Ameer Hassan, Audrius Širvinskas, Eugene Lin, Marc Ribo, Jordi Blasco, Muhammad Asif Taqi,
Aamir Badruddin, Adnan H Siddiqui, Timothy R Miller, Shazam M Hussain, Diogo C Haussen, Keith Woodward, Christoph Groden, Arturo Consoli,
M Imran Chaudry, Christian Ramsey, Alberto Maud, Joshua Bentley, Arsida Bajrami, Maher Sahnoun, Jens Fiehler, Rishi Gupta
Journal:
Journal of Neuro-Interventional Surgery– DEC 2023Background:
Challenges to revascularization of large vessel occlusions (LVOs) persist. Current stent retrievers have limited effectiveness for removing organized thrombi.
The NeVa device is a novel stent retriever designed to capture organized thrombi within the scaffold during retrieval.
Objective:
To evaluate the safety and effectiveness of revascularization of acute LVOs with the NeVa device.Methods:
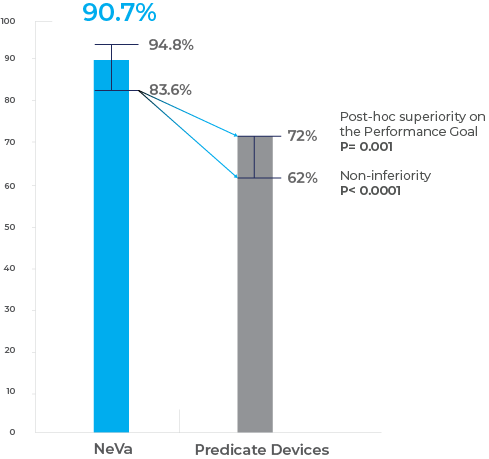
Prospective, international, multicenter, single- arm, Investigational Device Exemption study to evaluate the performance of the NeVa device in recanalizing LVOs including internal carotid artery, M1/M2 middle cerebral artery, and vertebrobasilar arteries, within 8 hours of onset. Primary endpoint was rate of expanded Treatment in Cerebral Ischemia (eTICI) score 2b–3 within 3 NeVa passes, tested for non- inferiority against a performance goal of 72% with a −10% margin. Additional endpoints included first pass success and 90- day modified Rankin Scale (mRS) score 0–2. Primary composite safety endpoint was 90- day mortality and/or 24- hour symptomatic intracranial haemorrhage (sICH).Results:
- From April 2021 to April 2022, 139 subjects were enrolled at 25 centers. Median National Institutes of Health Stroke Scale (NIHSS) score was 16 (IQR 12–20).
- In the primary analysis population (n=107), eTICI 2b–3 within 3 NeVa passes occurred in 90.7% (97/107; non- inferiority P<0.0001; post hoc superiority P<0.0001).
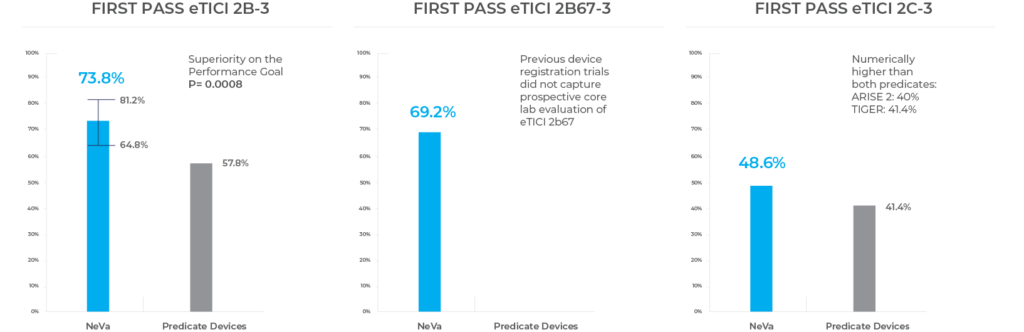
First pass recanalization was observed
- eTICI 2b–3 in 73.8% (79/107)
- eTICI 2b67–3 in 69.2% (74/107)
- eTICI 2c–3 in 48.6% (52/107)
Median number of passes was 1 (IQR 1–2)
- Final recanalization rates:
- eTICI 2b–3 in 99.1% (106/107);
- eTICI 2b67–3 in 91.6% (98/107);
- eTICI 2c–3 in 72.9% (78/107).
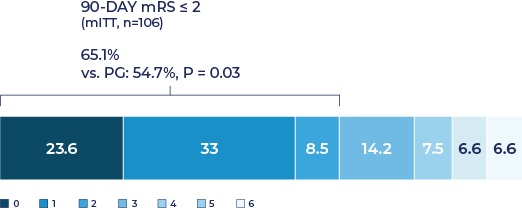
- Good outcome (90- day mRS score 0–2) was seen in 65.1% (69/106).
- Mortality was 9.4% (13/138) with sICH in 5.0% (7/139).
Conclusion:
The NeVa device is highly effective and safe for revascularization of LVO strokes and demonstrates superior first pass success compared with a predicate performance goal.