Authors:
Rishi Gupta, Keith Woodward, David Fiorella, Henry H Woo, David Liebeskind, Donald Frei, Adnan Siddiqui, Reade De Leacy, Ricardo Hanel, Lucas Elijovich, Alberto Maud for the VITAL Study Investigators
Journal:
Journal of NeuroInterventional Surgery
Background:
Cerebral vasospasm (CV) after aneurysmal subarachnoid hemorrhage (aSAH) is linked to worse neurological outcomes. The NeVa VS is a novel cerebral dilation device based on predicate stent retrievers. We report the results of the Vesalio NeVa VS for the Treatment of Symptomatic Cerebral Vasospasm following aSAH (VITAL) Study.
Methods:
This was a single-arm, prospective, multicenter trial to assess the safety and probable benefit of the NeVa VS device to treat CV. Patients were screened and treated if they had CV >50% on non-invasive imaging confirmed by cerebral angiography. The vessel diameters were measured before and after treatment by an independent core laboratory. The primary endpoint was ≥50% vessel diameter immediately after treatment with the NeVa VS device.
Results:
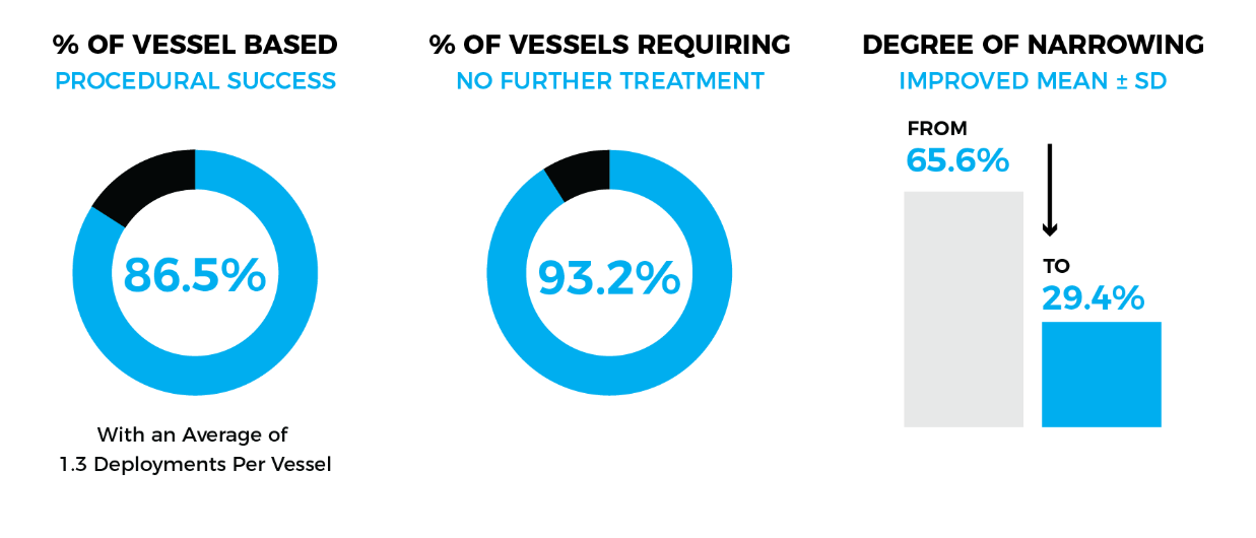
- 30 patients with a mean age of 52±11 years and mean Hunt–Hess grade of 3.1±0.9 were enrolled.
- A total of 74 vessels were treated with an average of 1.3 deployments per vessel (95 deployments total).
- The primary endpoint was achieved in 64 of 74 vessels (86.5%).
- The mean pre-treatment narrowing of the target vessel (n=74) was 65.6% with reduction of the narrowing to 29.4% after treatment.
- In 3/95 total deployments (3.2%), thrombus was observed at the site of deployment during the procedure without apparent neurological sequelae.
Conclusion:
The NeVa VS device appears to be a safe treatment to regain vessel diameter in severely narrowed intracranial arteries secondary to CV associated with aSAH. This treatment offers a new tool that allows for controlled vessel expansion to treat CV.