Paper:
Preclinical Evaluation of the NeVa™ Stent Retriever: Safety and Efficacy in the Swine Thrombectomy Model
Authors:
Ulm, Kachatryan, Grigorian, Nogeira
Journal:
INTERVENTIONAL NEUROLOGY, FEB 13, 2018
Background and Purpose:
NeVa stent retriever has been developed with an enhanced radial force profile, enlarged offset openings, and a closed distal end. The aim of this study is to evaluate the safety and effectiveness of this device in animal model of thrombo-occlusive disease.
Methods:
- For the efficacy study:
- 35 vascular occlusions and retrievals were performed using emboli of variable sizes and morphologies in 7 swine.
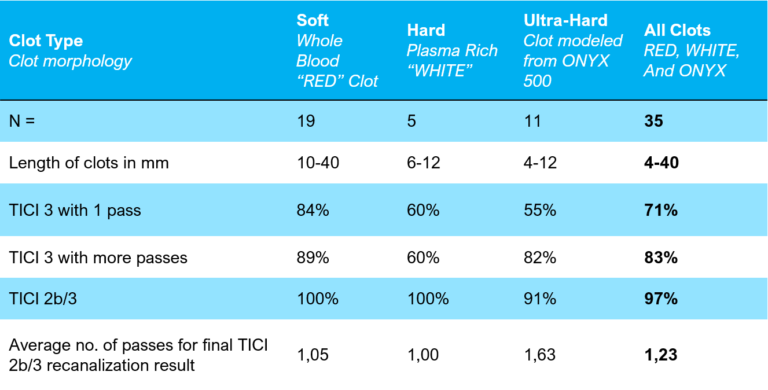
- Most previously published animal data on thrombectomy devices utilized autologous “red” whole blood clots of 10 to 15 mm
- For stent retrievers, efficacy of clot removal has been postulated to be dependent on the degree of migration of clot into the stent struts. It has also been postulated that most failures are related to the presence of a large, organized clot. Therefore, in the current study, 4 emboli morphologies with sizes ranging from 4 to 40 mm were tested, including 2 distinct models of autologous whole blood thrombi, plasma-enriched thrombi, and Onyx® emboli.
- 35 vascular occlusions and retrievals were performed using emboli of variable sizes and morphologies in 7 swine.
- Pre and post mTICI scores, number of retrievals, and the presence of angiographic complications were recorded.
- For the efficacy study:
- For the safety study:
- 6 clot retrievals were done and the vascular territory examined for injury and harvested for histopathological evaluation.
- A semiquantitative vasospasm study was performed in a separate animal
- Radial force testing was performed on NeVa and control devices for comparison.
Efficacy Analysis:
NeVa Overall Results: TICI 2b/3 = 97% with average 1.2 passes.
Safety:
Predicate Devices = Medtronic / Solitaire, Stryker / Trevo
NeVa Expansive Radial Force was higher than predicate devices. Interestingly, the difference was greatest at the largest diameter tested and smallest at the 2.0-mm lower limit.
NeVa Compressive Radial Force was comparable to predicate devices.
Histopathological Vessel Injury with NeVa was comparable to what was observed with predicate devices: the novel hybrid cell design and increased expansive radial force did not lead to unexpected vessel injury.
The vasospasm study demonstrated findings comparable with predicate devices
Conclusion:
NeVa device has unique properties and preclinical results support its use in a clinical trial to determine if its singular design improves outcomes in acute stroke.