Changing Outcomes
Treating Stroke and Vascular Occlusions
Stroke and cardiovascular disease, the biggest global killers, account for close to a third of annual deaths worldwide. Vascular occlusions from heart attacks and strokes claim an estimated 30 lives every minute.
Vesalio innovates to improve care for patients with vascular occlusions. Through state-of-the-art technology, Vesalio empowers physicians with tools to elevate clinical outcomes and change lives.
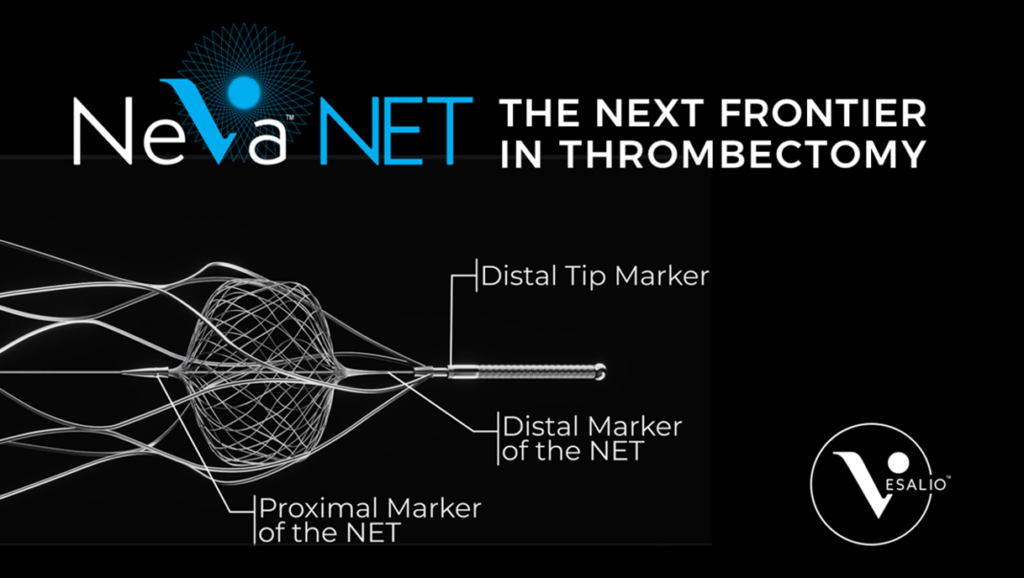
SPOTLIGHT ON NEVA™
NEVA CLEAR RESULTS PUBLISHED
Primary results from the prospective, international, multicenter FDA IDE study CLEAR demonstrated impressive clinical outcomes with Vesalio’s NeVa™ thrombectomy device in treating acute ischemic stroke caused by large vessel occlusions.
Prof. Laurent Spelle (Paris, FRANCE), Prof. Raul Nogueira (Pittsburgh, U.S.), and Prof. Serdar Geyik (Istanbul, TURKEY) discuss the results of the CLEAR trial.
CLOT BURDEN:
A BROAD ANATOMICAL CONCERN
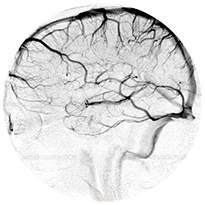
Neurovascular
Clots block circulation to an artery in the brain causing damage and loss of neurological function
AIS
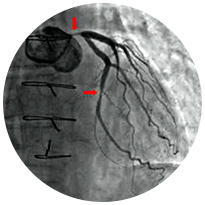
Cardiovascular
Clots restrict blood flow to a major artery in the heart, causing a heart attack and muscle damage
STEMI
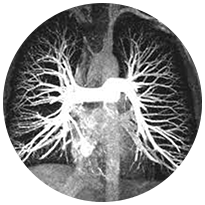
Peripheral Vascular
Clots can block flow anywhere in the body resulting in additional acute conditions such as
Pulmonary Embolisms
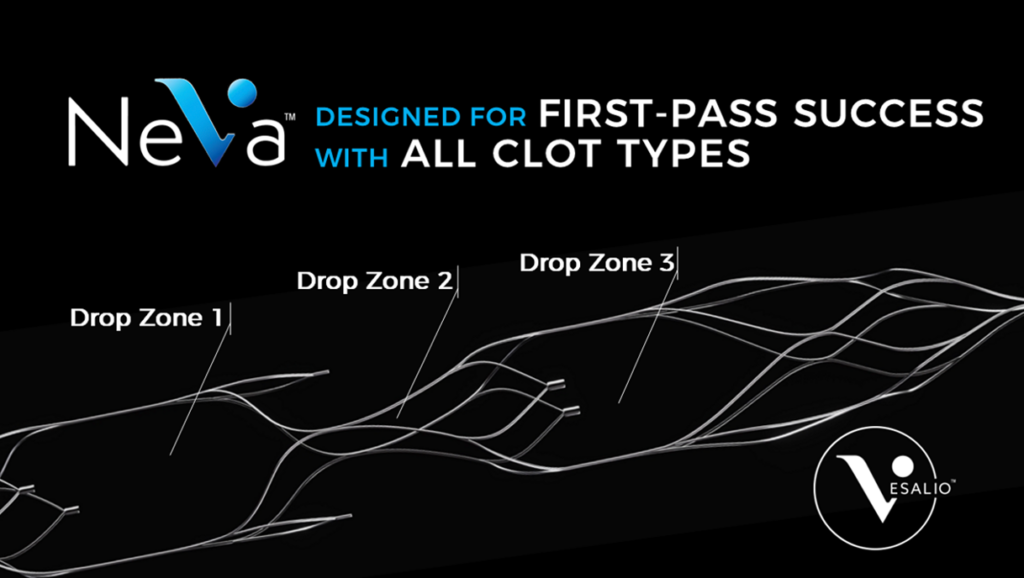
TAKING CLOT CAPTURE TO THE NEXT LEVEL
WITH DROP ZONETM TECHNOLOGY
Clots vary in density, size, and composition. Adding to the challenge, physicians cannot ascertain clot type before an intervention.
Vesalio’s Drop Zone™ Technology with its ALL CLOTS CAPABILITY brings substantial advantages to physicians and patients in thrombectomy procedures.
A SPECIFIC SOLUTION
FOR POST A-SAH VASOSPASM
Vasospasm is a frequently occurring and dangerous complication of aneurysmal subarachnoid hemorrhage (a-SAH).
Vesalio’s NeVa VS is the first and only FDA HDE approved device for the adjunct treatment in this indication.
Find out more: